Introduction to Validation for the (Bio)Pharmaceutical Industry programme provides an overview of essential validation activities from initial process design through to the finished commercial product. Focusing on key validation protocols and the validation life cycle, the programme explains the importance of validation in ensuring that all processes and manufactured products meet the required quality standards.
Aims and Objectives
The aim of this programme is to provide learners with an understanding of the regulatory requirements related to validation practices and documentation, to ensure consistent, high-quality pharmaceutical products. On completion of the programme, learners will be able to demonstrate knowledge and understanding of key validation protocols related to Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). Participants will also gain knowledge of validation documentation and the validation life cycle.
Course Content
The lifecycle approach to drug development processes and validation
Introduction to the key protocols within equipment validation - installation, operational and performance qualification (IQ, OQ, and PQ)
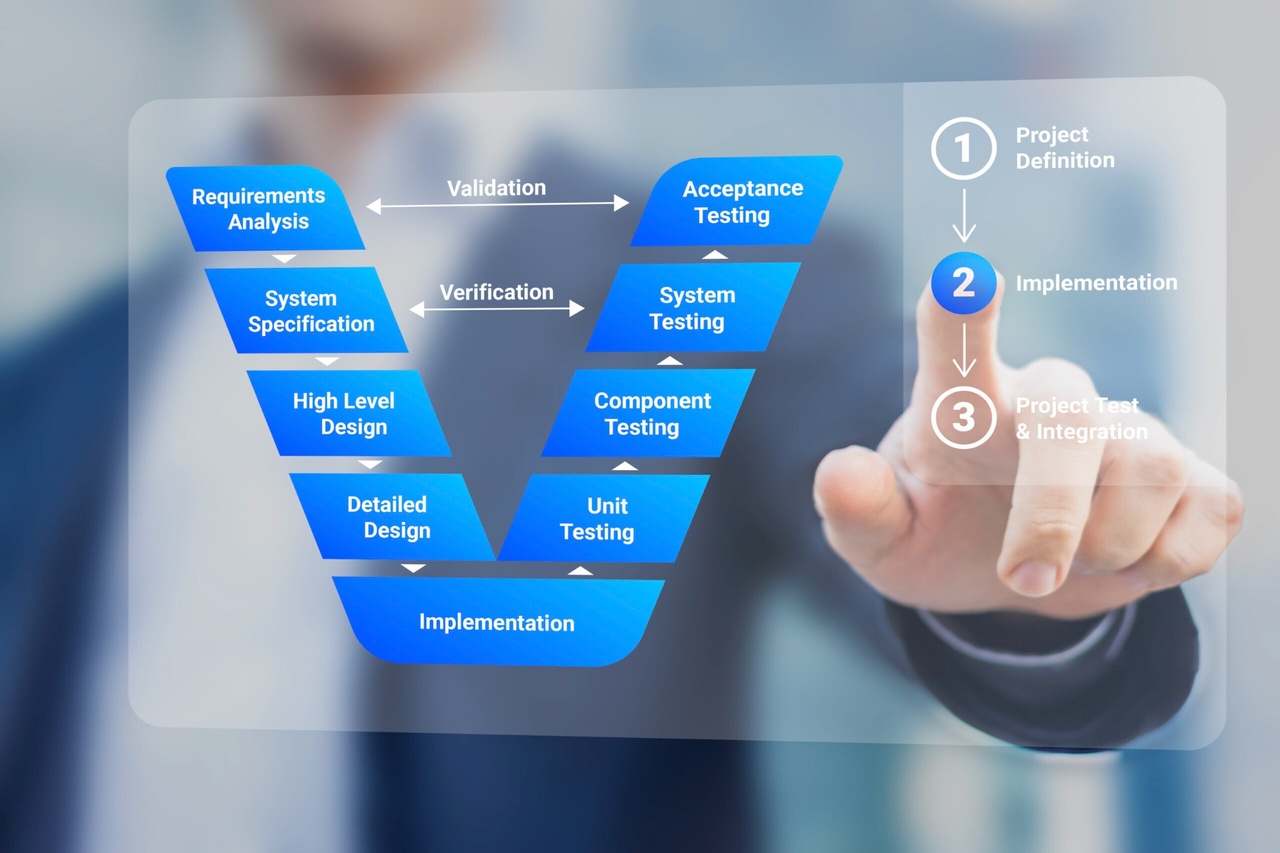
V Model for Validation
Validation Master Plan
Validation Documentation
Corrective Actions and Preventative Actions (CAPA)
Learning Outcomes
On completion of this programme the learner should be able to:
- Develop an understanding of the validation lifecycle
- Demonstrate knowledge and competence related to the V model for equipment qualification
- Demonstrate an awareness of the regulatory responsibility of a manufacturing organisation in relation to validation
- Understand the CAPA process in relation to validation studies
Other courses options include
- Introduction to Technical Writing for the Manufacturing Sector
- Advanced Technical Writing for the Manufacturing Sector
- Introduction to cGMP for the Bio-Pharmaceutical Industry
- Introduction to Clinical Trials for the Pharmaceutical Sector
- Introduction to Quality Control for Bio-Pharmaceutical Manufacturing
- Certificate in Supply Chain (Special Purpose Award/QQI Level 6 20 credits)
- Certificate in Operational Excellence (Special Purpose Award/QQI Level 6 25 credits)



















