- Overview
- Course outline
- Entry
- Career path
- How to apply
Course Overview
This innovative BSc in Process Technologies is a unique blend of MedTech, Pharma, and Food process technology modules. This course provides students with the most up-to-date and relevant skills to advance their careers in operator, technician, or supervisory roles within high-growth sectors.
This course will help students deepen their knowledge of good manufacturing practices (GMP) and regulations in the BioPharma, MedTech, and Food industries as well as focus on emerging trends within these sectors. Students will develop their skills in critical thinking, lean operations, technical writing & reporting, communications and presentations, and other areas that industry companies seek.
Integrating theoretical and practical learning in a Pilot Plant facility, graduates will experiment with analytical and biochemical techniques and understand their application within each sector.
- Medical Device Manufacturing
- Bioprocessing Manufacturing Technology
- Technical Writing
- Bio-Organic Chemistry
- Team Lead Skills & Professional Development
- Analytical Techniques
- Food Science & Health
- Lean Operations Project
Developed and taught by experienced academics and industry lecturers, this course is delivered through blended learning, 2 evenings per week online and up to 2 Saturdays per month in person.
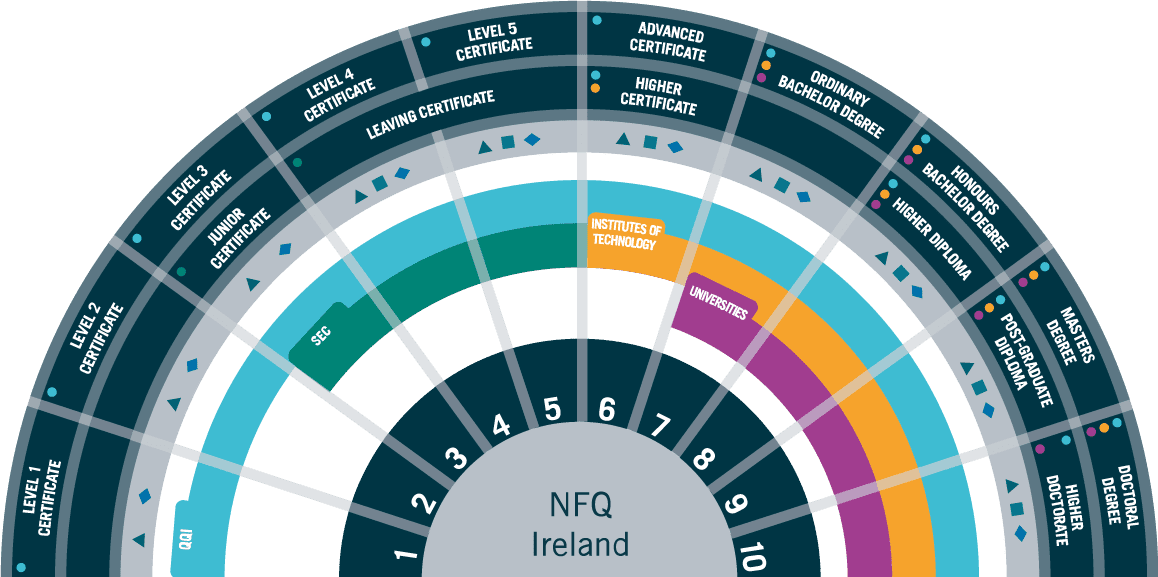
Accreditation: QQI Level 7 Bachelor of Science in Process Technologies
Collaborating University: Technical University Dublin – Tallaght Campus
Next Intake: January 2026. Now accepting applications.


Course Outline
The course duration is 11 months. Lectures will be delivered 2 evenings per week online. Students will also be required to attend 2 Saturdays a month on campus. Practicals will be in one of our pilot facilities, with dates and times subject to scheduling. (ECTS: 60 Credits)
This course will cover the following modules.
Module 1: Medical Device Manufacturing
Medical device manufacturing processes, regulation, product & process qualification/validation, cleanroom operations and product sterilization.
Module 2: Bioprocessing Manufacturing Technology
Upstream and Downstream processing operations, critical control parameters to produce a quality product, cleaning and impurity control precautions.
Module 3: Technical Writing
Effective communication, technical report writing, research methods and presentation skills.
Module 4: Bio-Organic Chemistry
Organic chemistry, biological systems, pathways and mechanism of important nutritional and pharmacological teams.
Module 5: Team Lead Skills & Professional Development
Organisational effectiveness, professional development, leading and managing teams, and working with diverse and geographical teams.
Module 6: Analytical Techniques
Raw material testing, in-process testing and final product release. Hands-on experience with key techniques such as IR, UV, HPLC, GC and TLC.
Module 7: Food Science & Health
Nutrition, human, physiology and diet-health relationship, health studies, current market and future trends.
Module 8: Lean Operations Project
Lean principles, practical application of the lean tool to identify and improve business processes.

Entry Requirements
Applicants are required to hold a minimum of a QQI Level 6 Certificate (120 credits) in Science, Engineering, Quality or related discipline.
Career Options
Over 70% of graduates have successfully advanced their careers post completion of our courses. Potential career opportunities following the successful completion of this course are highlighted below:
- Production Technician / Operator
- Maintenance Technician
- Manufacturing Supervisor/Team Leader
- Process Improvement Specialist
- Quality Assurance / Quality Control
- Supply Chain Planner
- Regulatory Compliance
- Health & Safety Officer
An essential part of the course will be the career progression module where significant support will be given to participants on career development through structured one-on-one CV assistance, interviewing support and developing career networks. regular guest speakers and lecturers from the industry will assist graduates in making links with the industry.
Apply Now
Our admissions team are on hand to assist you with your application and answer any questions you may have about the course.
Students who wish to apply to this course should follow the following application process.
Step 1: Enquire through the form at the top right of this page
Step 2: A member of our team will be in contact through phone or email
Step 3: If you are deemed to be eligible for the course, you will be sent an application form by email. You MUST fill in this application form and attach all necessary *documents (CV, ID, Transcripts, etc.)
Step 4: On submission of the completed application form and all documentation, your application will be sent to the associated department for final approval. You will receive an e-mail confirming your place on the programme. (Note: This process can take up to one week, particularly during busy admission periods.)
*We accept scanned documents in a pdf format. Pictures of documents are not accepted.
**For those who are applying for Springboard funding, you will be directed to fill out an additional application form on the Springboard website, this is to confirm your eligibility to receive funding
Please note at any time if you have any questions please do not hesitate to contact us by email on admissions@innopharmalabs.com or call us (01) 485 334
Why up-skill for the STEM process manufacturing sectors in Ireland?
- Over 130,000 employed within these industries
- Over 40,000 new jobs predicted
- Over €62 billion in exports
- 9 of top 10 world’s biopharma companies in Ireland
- 8 of the top 10 worlds medtech companies in Ireland
- Over 8,000 new jobs predicted

Testimonials
The course had online lectures so anytime I couldn’t go to college I could join the class online. I never missed a class.
BA in Pharmaceutical Business Operations Graduate, Joanna Galon, Senior Associate Regulatory Affairs at Amgen
Our Blog
Blog
January 22, 2026
Rethinking “Human Error” in Biopharmaceutical Manufacturing
Author: Ann Ryan, PhD Researcher, Director of Industry Engagement & Training, Innopharma Education A recent publication from A...
Blog
April 3, 2025
AI in Biopharma: Transforming Manufacturing & Innovation
How AI, Machine Learning, and Automation are Transforming Biopharmaceutical Manufacturing Here at Innopharma, we are proud to show...
Blog
December 11, 2024
Looking for a Career in the Biopharmaceutical Industry? Everything You Need to Know
The biopharmaceutical industry is a rapidly growing sector that combines cutting-edge biotechnology with pharmaceutical science to...






















